Chemical Procedures and Hair Breakage
Chemical procedures such as hair permanent hair dyes, bleaching, relaxers and perms can cause hair breakage. Below briefly describes common chemical procedures and how they could potentially cause hair breakage.
1.Permanent Hair dyes
Permanent colour contains an oxidising (hydrogen peroxide) agent, an alkalizing agent (ammonium hydroxide) and a pigment. The ammonium hydroxide opens up the cuticle by raising the pH to 8-9.5 allowing the pigment molecules to enter into the hair shaft. Ammonia also decomposes hydrogen peroxide into oxygen. The oxygen released from the hydrogen peroxide breaks down the melanin in the hair producing oxy-melanin, which is colourless. Large pigment molecules are produced when the pigment comes into contact with the oxygen released from the hydrogen peroxide. These large molecules of pigment are unable to leave the hair shaft and this results in permanent hair colour within the hair shaft.
A) Melanin+ Nascent Oxygen (released from hydrogen peroxide) =Oxy-Melanin (colourless)
→
B) Oxy-Melanin + Pigments (from hair dye) =Permanent hair colour.
Figure. 1
The potential hair damage from permanent hair colouring arises when it is done too often or done without subsequent conditioning afterwards or carried out on weak hair to start with. The hair can become dry and brittle which can result in hair breakage.
2. Bleaching
Hydrogen peroxide is used to de-colourise the natural pigments in the hair. Ammonium hydroxide is also used in the process to speed up the release of oxygen from the hydrogen peroxide and to swell and open up the cuticle to allow the hydrogen peroxide to enter the hair shaft.
The damage done to hair during bleaching is listed below:
• Permanent loss of disulphide Bonds
Oxidation of cystine to cysteic acid in the disulphide bonds results in reduced tensile strength of the hair, which makes it more susceptible to breakage.
• Cuticle damage
If overlapping occurs during retouching then cuticle damage can occur. The cuticle can become rough and break away resulting in weak porous hair.
• Breakage of Polypeptide chains
Over bleaching can break polypeptide bonds in the hair which will result in hair shaft breakage.
3. Perming
During the perming process, hair bonds are broken through a chemical reaction called reduction. The reducing agents used in perms are called thiol compounds, usually ammonium thioglycollate is used. Another chemical used in perming is ammonium hydroxide, this opens up the cuticle to allow the thioglycollate to penetrate the hair shaft.
Once thioglycollate is in the cortex of the hair, it breaks the disulphide bonds in the hair by adding hydrogen atoms. The broken bonds are then reformed around perm rods (rollers) by oxidation using hydrogen peroxide.
Hair breakage during and after perming can be a result of the following:
• Cuticle damage caused when the ammonium hydroxide is applied the hair.
• Loss of protein during over- oxidation of the hair and lack of subsequent conditioning.
• Too much tension applied to the hair as it is curled around the rollers.
• Over-processing (reduction) results in too many cystine bonds broken. These broken bonds weaken the hair and make it susceptible to breaking.
• Over-neutralising with hydrogen peroxide results in cystine changing to cysteic acid which does not form linkages.
4. Relaxing
There two types of relaxers available, one type contains sodium hydroxide also known as lye relaxers and the other ‘No lye’ relaxer is based on two ingredients; calcium hydroxide and guanidine carbonate. The two ingredients in ‘No Lye’ relaxers are added together to form the alkaline relaxer (see fig 2 below).
Calcium hydroxide + guanidine carbonate →guanidine hydroxide (alkaline relaxer) + calcium carbonate
Fig.2
Relaxers break the disulphide bonds by a process called hydrolysis. The hair is permanently straightened and left very alkaline. When the relaxing process is complete the relaxer must be rinsed off using an acid balanced shampoo to neutralise the hydroxide ions and lower the pH of the hair.
Relaxers are very harsh alkaline chemicals which can cause breakage of the hair because of the following reasons:
• Relaxed hair is torsionally stressed and can break easily.
• Incomplete rinsing of the hair could leave the hair alkaline and result in dry brittle hair that breaks easily.
• Incorrect relaxing solutions and timing mistakes could also cause hair breakage. If relaxers are too alkaline and left on the hair too long they could have a depilatory action.
• Lack of conditioning treatments following relaxing.
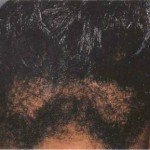
Figure 3 on the left shows hair breakage on a black person who has had their hair relaxed.
The chemcials used in these procedures can be seriously damage your hair. Please make sure if you have any of the above procedures that they are carried out by a fully qualified professional.
J Norris-property of Roots2Ends

